According to the British Daily Mail, as the most abundant element in the universe, hydrogen has the potential to fuel our cities. It only produces water and heat. Although this seems like a very promising technology, artificially creating hydrogen has always been considered very difficult and costly. A team of engineers now proposes a solution: a low-cost water separator that continuously produces hydrogen and oxygen.
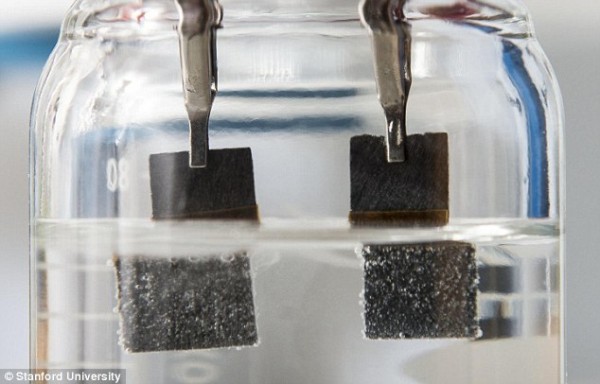
Two electrodes immersed in a water-based electrolyte
Traditional hydrolyzer devices consist of two electrodes submerged in a water-based electrolyte. The low-voltage current applied to the electrodes drives the catalytic reaction that decomposes water molecules, producing hydrogen bubbles in one electrode and oxygen bubbles in the other. Each electrode is embedded with different catalysts, typically platinum and iridium, which are two rare but expensive metals.
But last year, Hongjie Dai, a chemist at Stanford University in the United States, invented a hydrolyser that consists of inexpensive nickel and iron. They only need ordinary 1.5-volt batteries. This latest equipment further promotes the development of water electrolysis technology. “Our water disintegrators are very unique because they use only one catalyst, nickel-iron oxide, for both electrodes,†says Haotian Wang, a graduate student at Stanford University. "This dual-function catalyst only needs to rely on a stable 1.5 volt electrical input to continuously decompose water for more than one week. It can achieve an unprecedented 82% water decomposition efficiency at room temperature."
In conventional hydrolyzers, hydrogen and oxygen catalysts often require different electrolytes with different pH values ​​- one acidic and one alkaline - to remain stable and active. "For the actual water decomposition, an expensive barrier is often required to separate the two electrolytes, which further increases the cost of this equipment."

This process creates closely connected small particles, so the catalyst has very good conductivity and stability
“But our single-catalyst hydrolyser requires only a single electrolyte with the same pH to operate efficiently.†Wang and his colleagues found that nickel-iron oxides—which are very cheap and easy to produce—are actually more expensive than some. Commercial catalysts consisting of metals are more stable. “Decomposing metal oxides into small particles increases the surface area and exposes a large number of extremely small, interconnected particle boundaries that will become active sites for the decomposition of water-catalyzed reactions.†Cui Yi, Associate Professor, Materials Science and Engineering, Stanford University Yi Cui) expressed this.
"This process creates closely linked small particles, so the catalyst has very good electrical conductivity and stability." The use of a catalyst consisting of nickel and iron is of great significance from a cost perspective. "Not only is the material cheaper, just using a single catalyst will reduce the two sets of capital investment to one set," Cui explained. "We believe that electrochemical regulation can be used to find new catalysts for chemical fuels other than hydrogen."
Material: brass
Surface finish: Polishing (mirro), chromed plated (mirror)
Surface roughness: Rz 3.2
Casting minimum tolerance: +/-0.2mm
Inspection: Slide caliper, microcaliper, projector, 100%
Certification: CE,ACS,Watermark
OEM Service: We can produce it according to your requirement.
Package: bag,inner box,outbox
Brass Spout,Brass Faucet Spout,Brass Round Spout,Brass Swivel Spout
KaiPing HuiPu Shower Metalwork Industrial CO,LTD , https://www.hp-shower.com