Abstract: The microstructure and phase composition of the brazing joints of 4 nickel-based brazing materials 1Cr18Ni9Ti and their changes with heat treatment time were systematically studied by metallographic test, X-ray diffraction phase analysis and electron probe analysis. The results show that the braze joint with a slit width of 0.10 mm brazed with BNi1a and BNi2 (1 120 °C, 10 min) consists of α-Ni, Ni 5 Si 2 , CrB and Fe 4.5 Ni 18.5 B 6 , near The slit diffusion layer is composed of α-Fe, γ-Fe and Fe 2 B. A brazed joint having a slit width of 0.05 mm brazed with BNi5 solder (1 175 ° C, 10 min) consisted of α-Ni, Ni 5 Si 2 and Ni 16 Cr 6 Si 7 . Brazing joints with a seam width of 0.05 mm brazed with BNi7 solder (1050 ° C, 10 min) consist of α-Ni, Ni 2 P and (Fe,Ni) 3 P.
Introduction
   1Cr18Ni9Ti stainless steel is one of the most widely used materials in the industry. Brazing stainless steel with nickel-based brazing material has a great influence on the properties of the brazing joint. In 1978, R. Johson [1] used electron probes to analyze the microstructure of brazed joints of 9Cr-1Mo steel brazed with BNi - 2 and BNi-4. Since the electron probe can only perform semi-quantitative analysis on boron, the measured data has low precision, and it is difficult to determine the boron compound phase. In this paper, metallographic analysis and electron probe analysis were combined with X-ray diffraction phase analysis to study the phase composition of the brazing joint and the elimination of the compound phase in the brazing joint.
1 Test materials and test methods
   The base material is 1Cr18Ni9Ti, and the chemical composition of the solder is listed in Table 1.
Table 1 Chemical composition of test solder
| Solder category | chemical composition(%) | Melting point T/°C | ||||||
| Ni | Cr | B | Si | Fe | P | Solid line | Liquidus | |
| BNi1a | margin | 14 | 3.1 | 4.5 | 4.5 | - | 977 | 1 097 |
| BNi2 | margin | 7 | 3.1 | 4.5 | 3.0 | - | 971 | 999 |
| BNi5 | margin | 19 | - | 10 | - | - | 1 073 | 1 135 |
| BNi7 | margin | 13 | - | - | - | 10 | 888 | 888 |
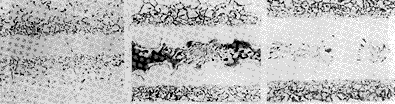
| The surface of the sample was polished with a grinder, and the width of the brazing joint was adjusted by using a stainless steel gasket added at both ends of the brazing joint, and positioned by argon arc welding. The brazing test was carried out in a hot wall vacuum furnace, and the solder used was powdered and placed directly on the sample for brazing. The degree of vacuum during brazing is better than 0.01 Pa. The phase composition of the braze joint is determined by metallographic analysis and electron probe analysis combined with X-ray diffraction phase analysis. 2 brazing joint structure and phase composition analysis 2.1 BNi1a solder    The 1Cr18Ni9Ti was brazed with a BNi1a solder. Under the conditions of a slit width of 0.05 mm, a brazing temperature of 1120 °C and a brazing time of 10 min, the brazed joint structure is a pure solid solution, as shown in Fig. 1(a). In the BNi1a solder, boron is a compound phase. The main element has a content of 3.1%. The solid solubility of boron in nickel is very low. A single solid solution structure indicates that most of the boron in the braze has spread to the base material during the brazing process. The amount of boron in the braze has been reduced below the limit solid solubility of boron in nickel. It can also be seen from Fig. 1 that the near-seam region in the base material is a network structure which is produced by the diffusion of boron in the brazing filler metal along the grain boundary of the parent metal. The interface of the grain boundary in the base metal has a relatively open structure, and the resistance to atomic motion is small, and the radius of the boron atom is relatively small. Thus, the diffusion of boron to the base material is mainly along the grain boundary and is in the crystal. The boundary forms a compound phase with other elements. X-ray diffraction analysis, phase composition near the weld zone is α-Fe, γ-Fe and Fe 2 B, i.e., boron and iron in the grain boundary is formed between the mutual diffusion of Fe 2 B. with the base metal so that the brazing joints The composition of the base material diffusion layer is changed, thereby producing α-Fe. Since the Fe 2 B is present on the grain boundary, the near-seam region in the base material is electrolytically etched to exhibit a network structure as shown in FIG. Fig. 1(b) shows the braze joint structure brazed with BNi1a solder (1 120 °C, 10 min) when the slit width is 0.10 mm. The brazed joint structure is composed of two parts: one part is a nickel solid solution structure which is close to and parallel to the interface between the base metal and the brazing seam; the other part is a compound phase structure which is located in the middle. The phase structure of the compound has two structural features: a plum-like compound phase and a block-like pitting compound phase. Since the brazing seam is wide, the boron content in the brazing seam cannot be reduced below the ultimate solid solubility by diffusion during the brazing time, thereby producing a boron compound phase in the brazing seam. It can be seen from the microstructure characteristics of the brazing joint that the distribution of boron in the brazing joint is not uniform. At the interface near the base metal and the brazing joint, the boron content has been reduced below the ultimate solid solubility due to diffusion, presenting a solid solution structure. In the middle of the brazing joint, the diffusion path is long, the diffusion speed is slow, and the boron content at the place after welding is still above the ultimate solid solubility, thereby forming a compound phase in the middle of the brazing seam. X-ray diffraction analysis showed that the phase composition was α-Ni, Ni 5 Si 2 , CrB and Fe 4.5 Ni 18.5 B 6 . After 1 h diffusion treatment at 1 000 °C, the blocky pitting compound phase in the brazing joint It disappears, leaving only the plum-like compound phase, as shown in Figure 1(c). X-ray diffraction analysis shows that the plum-like compound phase is CrB. It can be seen that the phase composition of the block-like pitting compound is Ni 5 Si 2 and Fe 4.5 Ni 18.5 B 6. The diffusion treatment time was extended to 1 h at 1 000 °C, and the CrB phase did not disappear. |
2.2 BNi2 solder |
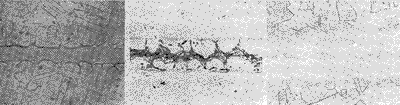 (a) (b) (c) Figure 2 Brazing joints brazed with BNi5 solder |
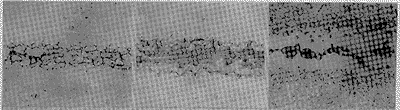 (a) (b) (c) Figure 3 Brazing joints brazed with BNi7 solder 3 Conclusion    (1) Under the brazing specification with brazing temperature of 1 120 °C and brazing time of 10 min, the brazed joint brazed with BNi1a brazing material is a single nickel solid solution with a slit width of less than 0.05 mm, and the slit width is larger than A compound phase may occur at 0.05 mm. Among them, CrB is relatively stable and difficult to eliminate during the post-weld diffusion treatment. (2) Under the brazing temperature of 1 050 °C, 1Cr18Ni9Ti is brazed with BNi2 solder, no CrB compound phase appears in the brazing joint, and the compound phase in the brazing seam can be eliminated by post-weld diffusion treatment. (3) Brazing (1 175 ° C, 10 min) 1Cr18Ni9Ti with BNi5 solder. When the slit width is 0.02 mm, the brazed joint structure is a pure solid solution. When the slit width is 0.05 mm, a large amount of compound phase appears in the brazing seam. After the diffusion treatment, most of the compound phase in the brazing seam is eliminated. (4) It is difficult to braze the brazed structure into a single solid solution structure by brazing (1 050 ° C, 10 min) 1Cr18Ni9Ti with BNi7 solder. At a slit width of 0.01 mm, there is still a compound phase in the weld after welding. |
Led Lantern,Outdoor Solar Lanterns,Battery Operated Lanterns,Solar Lantern Lights
Hangzhou Fantasy Technology Co.,ltd , https://www.ledluxlite.com